Alsonex
Alsonex – Developing a New Treatment for Motor Neuron Disease
The mission of Alsonex is to discover and develop new pharmaceutical products and technology through to proof-of-concept studies in areas where the clinical need is acute and to partner those products and technologies for further development and marketing. Alsonex has an expanding interest in neurodegenerative disease built on modulation of the innate immune system.
Inflammation
Inflammation is the immune response to injury to one of the organs of the body. It is most obvious and most immediately apparent when the injury is to the skin. Acute inflammation presents as pain, heat, swelling, and redness at the site of the injury and it may involve loss of function of the involved tissues. This type is normal and is the protective response following trauma or infection. Acute inflammation involves local dilation of blood vessels and increased vessel permeability to improve blood flow to the injured area. At the site of an infection or injury, a variety of cells release signalling molecules that recruit leukocytes to the affected area. However, inflammation that becomes chronic can damage the body’s own organs as the inflammatory cells release reactive oxygen species and proteases in an attempt to destroy the source of inflammation. Tissue damage is a characteristic of chronic inflammation. A common occurrence for this is in the disease asthma, where chronic inflammation of the lung leads to structural changes in the lung and, if left untreated, irreversible damage to the lung, loss of lung function with disastrous consequences for the patient. More often than not and particularly in the brain the body is unable to repair tissue damage and the cascade of events creates further damage. ALS is such a disease where neuronal inflammation is apparent. A key component driving this inflammation is a suite of proteins known as the complement system. The complement system is complex, but now well understood and, theoretically at least, a highly targeted inhibitor of the complement system should dampen down inflammation, return the brain to a more normal homeostatic condition and prevent further damage. ALS-205 is such an inhibitor of the complement system
Neurodegenerative Disease
Neurodegenerative diseases occur when nervous system cells (neurons) in the brain and spinal cord begin to deteriorate. Changes in these cells cause them to function abnormally and eventually result in the death of the cell. Neurons normally don’t reproduce or replace themselves, so when they become damaged or die they cannot be replaced. As neurons deteriorate, an individual may first experience relatively mild symptoms — problems with coordination or remembering names. But as larger numbers of neurons die, symptoms become progressively worse. In some cases, patients lose the ability to walk independently, think clearly, or generally function in the world. Ultimately, many of these diseases are fatal.
Examples of Neurodegenerative Diseases
Examples of neurodegenerative diseases include Amyotrophic Lateral Sclerosis (ALS), Parkinson’s, Alzheimer’s, and Huntington’s disease.
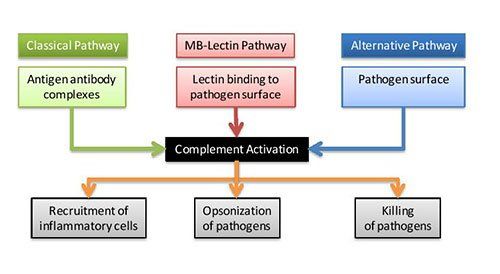
There are three main pathways of complement activation: the classical pathway, which is triggered by antibody or by direct binding of complement component C1q to the pathogen surface; the MB-lectin pathway, which is triggered by mannan-binding lectin, a normal serum constituent that binds some encapsulated bacteria; and the alternative pathway, which is triggered directly on pathogen surfaces. All of these pathways generate a crucial enzymatic activity that, in turn, generates the effector molecules of complement.
The three main consequences of complement activation are opsonization of pathogens (making them susceptible to destruction), the recruitment of inflammatory cells, and direct killing of pathogen
Complement is mostly activated by three distinct pathways as shown in the Figure. However, a fourth extrinsic pathway exists that helps amplify the cascade using cellular proteases to cleave C3 and C5.
Complement activation
Complement
The complement system is made up of a number of distinct plasma proteins that react with one another to adhere to pathogens and induce a series of inflammatory responses that help fight infection. A number of complement proteins are proteases that are themselves activated by proteolytic cleavage. In the complement system, the precursor proteins are widely distributed throughout the body without causing any problems. However, at sites of infection, they are activated locally and trigger a series of potent inflammatory events. The complement system activates through a triggered-enzyme cascade. In such a cascade, an active complement enzyme, generated by cleavage of its precursor protein cleaves its substrate, another complement protein, to its active enzymatic form. This, in turn, cleaves and activates the next protein in the complement cascade. In this way, the activation of a small number of complement proteins at the start of the pathway is hugely amplified by each successive enzymatic reaction, resulting in the rapid generation of a disproportionately large complement response. As might be expected, there are many regulatory mechanisms to prevent uncontrolled complement activation.
The three distinct pathways through which complement can be activated depend on different molecules for their initiation, but they converge to generate the same set of effector molecules. There are three ways in which the complement system protects against infection:
- Generating large numbers of activated complement proteins that bind to pathogens, making bacteria or other cells susceptible to the action of phagocytes bearing complement receptors.
- The small fragments of some complement proteins act as chemoattractants to recruit more phagocytes to the site of complement activation, and also to activate these phagocytes.
- The terminal complement components damage certain bacteria by creating pores in the bacterial membrane.
The Complement Cascade
Figure: The main pathways for complement activation.
The classical pathway is triggered by activation of the subunits of C1 after contact with immunoglobulin-G (IgG)- and IgM-containing immune complexes and by C-reactive protein (CRP).
The lectin pathway involves the interaction of a serum protein, mannose-binding lectin (MBL), with mannose residues on bacterial surfaces, resulting in the activation of MBL-associated serine protease 1 in the serum. At this point, the classical and lectin pathways converge, the outcome being limited cleavage of C4 and C2, generating C4bC2a, a C3 convertase.
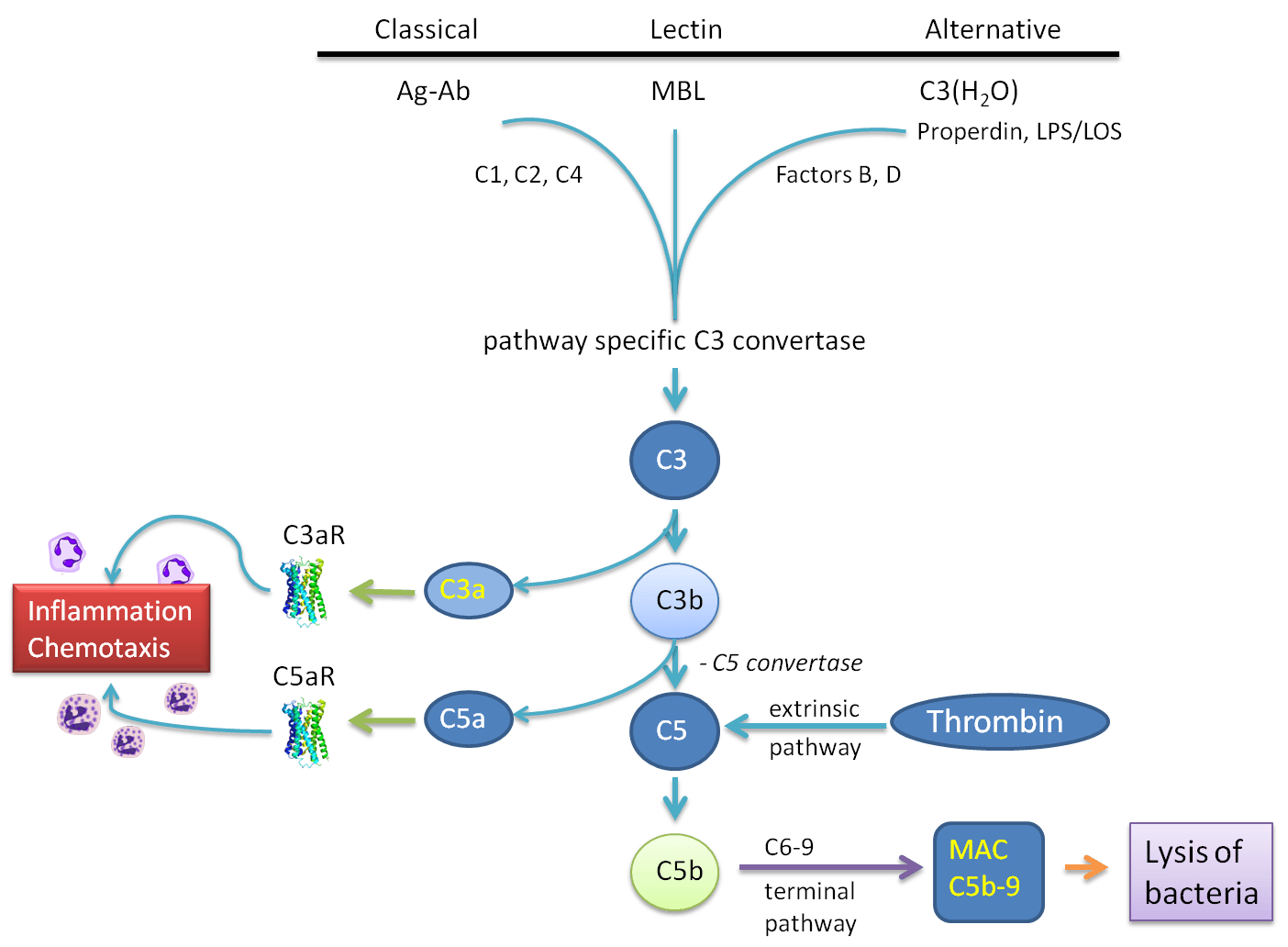
The alternative pathway is typically triggered by lipopolysaccharide (LPS) derived from Gram-negative bacteria. C3b is formed and interacts with factor B, followed by activation of factor D, resulting in the generation of a C3 convertase. C3 convertases is where the three main pathways converge and results in the limited cleavage of C3, generating the smaller fragment C3a, and the larger fragment C3b. C3a is an anaphylatoxin that causes smooth-muscle contraction, vasodilation and increased vascular permeability, which are all features of the acute inflammatory response. C3b is an important opsonic factor. C3b also becomes incorporated into the C3 convertase, forming a C5 convertase, which selectively cleaves C5 into C5a and C5b. C5a is a potent anaphylatoxin, and is the centrepiece of the complement cascade. The other fragment from C5 cleavage, C5b, interacts sequentially with C6, C7, C8 and C9 to form C5b–C9, the membrane-attack complex (MAC).
The small complement fragments C3a and C5a act on specific receptors to produce local inflammatory responses. When produced in large amounts or injected systemically, they induce a generalized circulatory collapse, producing a shock like a syndrome similar to that seen in a systemic allergic reaction involving IgE antibodies. Such a reaction is termed anaphylactic shock and these small fragments of complement are therefore often referred to as anaphylotoxins. C5a is the most stable and has the highest specific biological activity. Both C5a and C3a can induce smooth muscle contraction and increases vascular permeability and act on the endothelial cells lining blood vessels to induce adhesion molecules.
In addition, C3a and C5a can activate the mast cells that populate submucosal tissues to release mediators such as histamine and TNF-α that cause similar effects. The changes induced by C5a and C3a recruit antibody, complement, and phagocytic cells to the site of an infection and the increased fluid in the tissues hastens the movement of pathogen-bearing antigen-presenting cells to the local lymph nodes, contributing to the prompt initiation of the adaptive immune response.
Complement Plays a Central Role In Innate Immunity
Complement plays a central role being involved in innate immunity, driving local and central inflammation and in the destruction of neurons. Neuroinflammation, including microglial activation, reactive gliosis, and early activation of the classical complement cascade, is a hallmark of many neurodegenerative diseases. The recognition that the complement activation and activated microglia play an important role in eliminating synapses throughout the normal developing brain raises the possibility that complement activation actively drives the loss of synapses early in neurodegenerative disease, leading to loss of neurons and accelerating disease progression. The complement component C5a is also produced locally in the CNS and the C5a receptor is present on neurones and glia. In the case of MND, there is a significant contribution of immune and inflammatory components to the pathology of the disease. Activation fragments of the complement system are increased in the plasma and cerebrospinal fluid of MND patients. Elevated levels of these fragments are also localised to glia in the spinal cord and the motor cortex of these patients. C5a is also elevated within leukocytes from ALS patients suggesting heightened C5a receptor interaction. These findings indicate there is enhanced peripheral immune complement terminal pathway activation in MND, which likely plays an important role in the disease process.




